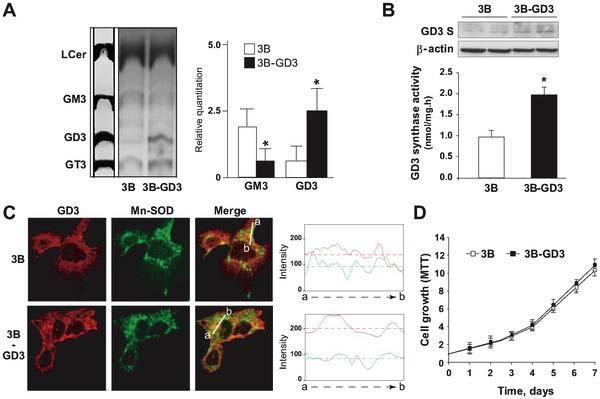
Characterization of GD3 synthase-expressing Hep3B cells. GD3 synthase overexpression in human hepatocellularcarcinoma Hep3B cells, alters the native pattern of glycosphingolipids featuring a predominant synthesis of GD3 from the endogenous GM3. GD3 synthase overexpression distributes to diverses intracellular structures, including mitochondria as revealed by the confocal imaging showing the colocalization of GD3 synthase (in red) with Mn-SOD (in green). This outcome, however, does not alter cell growth under normoxia, but sensitizes GD3 synthase overexpressing Hep3B cells to hypoxia. For more information, see the article by Lluis et al., PLoS ONE, 4, e8059, 2009.
Hepatocellular carcinoma is one of the leading causes of cancer-related death whose incidence is on the rise due to its association with obesity and nonalcoholic steatohepatitis. Currently the therapy of HCC is inefficient due to the high resistance of HCC to current cancer therapy. Therefore the uncovering of novel strategies to sensitize HCC to exisiting or future available therapeutic avenues is of outmost interest. Here we describe a novel role of ganglioside GD3 in HCC cell biology and therapy. The overexpression of GD3 synthase in HCC cells stimulates the synthesis of GD3 from endogenous GM3 and sensitizes HCC cells to hypoxia-mediated cell death and reduces in vivo tumor growth by disabling of survival pathway dominated by Src/NF-kB activation due to oxygen deprivation. These findings identify GD3 as a potential relevant therapeutic agent to turn hypoxia from a cancer-promoting environment to a cancer-threatening milieu. Full article published by Lluis et al, PLoS ONE 11, e8059, 2009