Alcohol-related liver disease (ALD) is a spectrum of liver disorders that begins with steatosis, a reversible stage of the disease that can progress to cirrhosis and liver cancer. Despite being one of the leading causes of chronic liver disease and morbidity in the world, the pathogenesis of ALD is still incompletely understood, which has limited the availability of effective therapies. The anatomical features and functional complexity of zone-restricted gene expression in the liver makes even more challenging to understand the mechanisms whereby chronic alcohol intake injure hepatocytes and sensitize to disease progression.
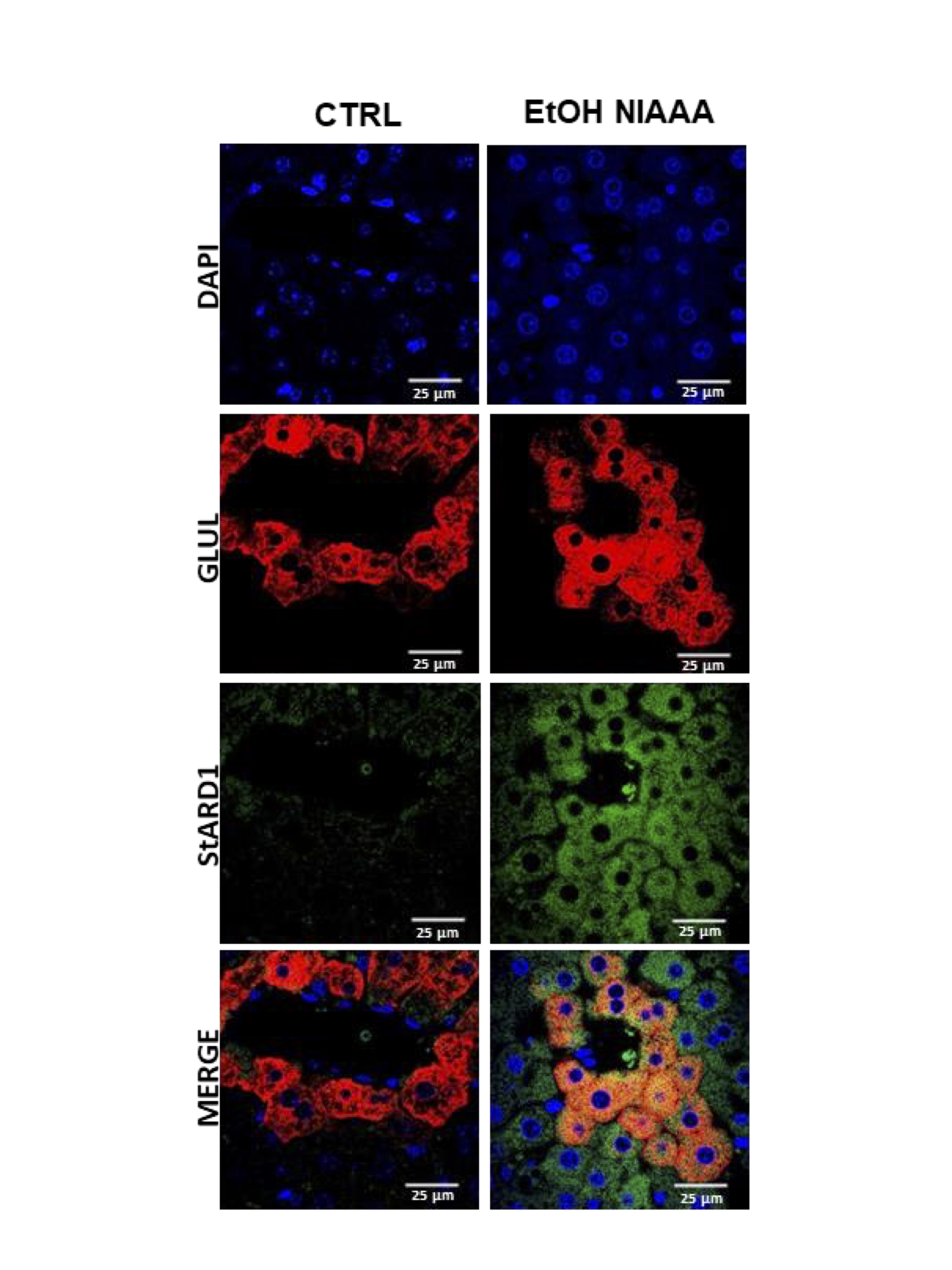
In a recent study published in the Journal of Lipid Research, the group of “Mitochondrial regulation of cell death“ reports that StARD1, a mitochondrial outer membrane protein that delivers cholesterol to mitochondrial inner membrane for metabolism, is predominantly expressed in perivenous (PV) zone of liver sections from mice fed chronic alcohol-containing diets. The zonal distribution of StARD1 is observed as early as 10 days of alcohol feeding in isolated PV compared to periportal (PP) hepatocytes and in the acute-on-chronic model of ALD. The zonal-dependent expression of StARD1 resulted in the accumulation of cholesterol in mitochondria and increased lipid peroxidation in PV hepatocytes from mice fed alcohol compared to PP hepatocytes. Transmission electron microscopy revealed structural zonal-dependent alterations in mitochondria. Extracellular flux analyses of real-time oxygen consumption rates through Searhorse indicated lower maximal respiration and spared respiratory capacity in control PV hepatocytes that were reversed upon alcohol feeding. More importantly, the differential morphology and functional activity of mitochondria between PP and PV hepatocytes following alcohol feeding is governed by StARD1 as mice with hepatocyte-specific StARD deletion (Stard1Δhep) are resistant to alcohol-induced ultrastructural and functional mitochondrial defects. Hence, this study identifies an additional factor that can contribute to the spatial, zonal-dependent sensitivity of the liver to the damaging effects of alcohol besides the known preferential metabolism of alcohol in the centrilobular area. Several factors converge in this area, such as lower oxygen tension and alcohol metabolism that synergize to induce StARD1, leading to subsequent mitochondrial cholesterol delivery that disrupts mitochondrial antioxidant defense and function. These new finding thus suggest StARD1 as a novel target for the treatment of ALD.
Article: Fucho et al. Zonal expression of STARD1 and oxidative stress in alcoholic-related liver disease. doi: https://doi.org/10.1016/j.jlr.2023.100413.