HCC is the most common type of liver cancer and the end-stage of chronic liver diseases of diverse etiologies, including viral hepatitis, alcoholic liver disease or nonalcoholic steatohepatitis (NASH). NASH-driven HCC is expected to increase worldwide due to its association with the obesity and type-2 diabetes epidemic.
Overweight and obesity are known risk factors for cancer development, especially HCC. Despite important improvements in the management of HCC that were made in the last 20 years, effective treatment options are mainly limited to early disease stages. Unfortunately, the therapeutic armamentarium for HCC is limited, ineffective and subject to secondary or acquired chemoresistance by poorly understood mechanisms.
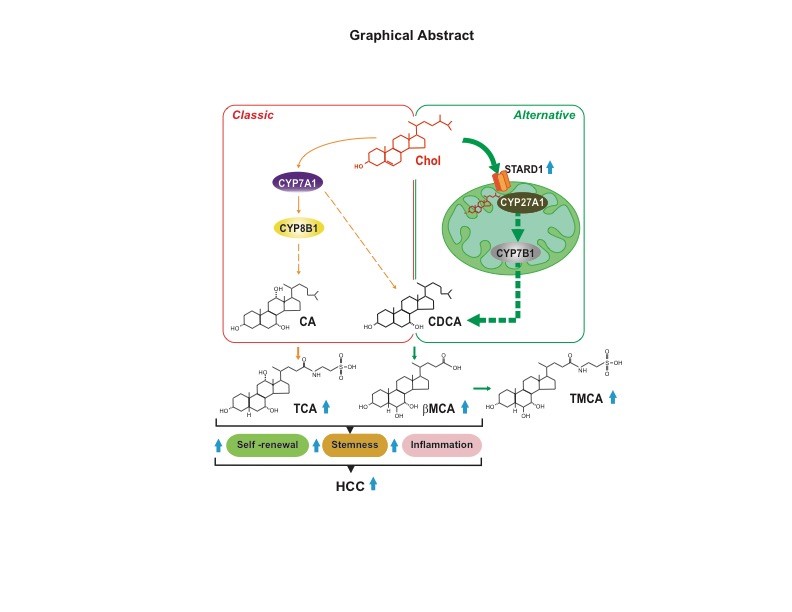
In a recent study published in the Journal of Hepatology, with Laura Conde de la Rosa as the first author, the investigators, coordinated by Jose C Fernandez-Checa leader of the group “Mitochondrial Regulation of Cell Death” of the IIBB-CSIC, in collaboration with Michael Karin (University of California San Diego) uncover a critical role for STARD1, a mitochondrial protein responsible for the trafficking of cholesterol to the mitochondria inner membrane, in NASH-driven HCC through the generation of bile acids (BAs) in the alternative mitochondrial pathway. In addition to their physiological role in bile formation and fat digestion, BAs act as signaling molecules and modulate liver tumorigenesis. Using NASH-driven HCC models, STARD1 overexpression in WT mice increased liver tumor multiplicity, whereas hepatocyte-specific STARD1 deletion (Stard1ΔHep) in WT or MUP-uPA mice reduced tumor burden. These findings mirrored the levels of unconjugated primary BAs in STARD1 overexpressing and Stard1ΔHep mice. Incubation of tumor-initiated stem-like cells with a mix of BAs mimicking this profile stimulated expression of genes involved in pluripotency, stemness and inflammation. Thus, these findings may have potential translational and clinical relevance as they identify STARD1 as a novel player in NASH-driven HCC by regulating the generation of BAs through the alternative pathway.
Article:
https://doi.org/10.1016/j.jhep.2021.01.028
Conde de la Rosa L, Garcia-Ruiz C, Vallejo C, Baulies A, Nuñez S, Monte MJ, Marin JJG, Baila-Rueda L, Cenarro A, Civeira F, Fuster J, Garcia-Valdecasas JC, Ferrer J, Karin M, Ribas V, Fernandez-Checa JC. STARD1 promotes NASH-driven HCC by sustaining the generation of bile acids through the alternative mitochondrial pathway. J Hepatol, in press