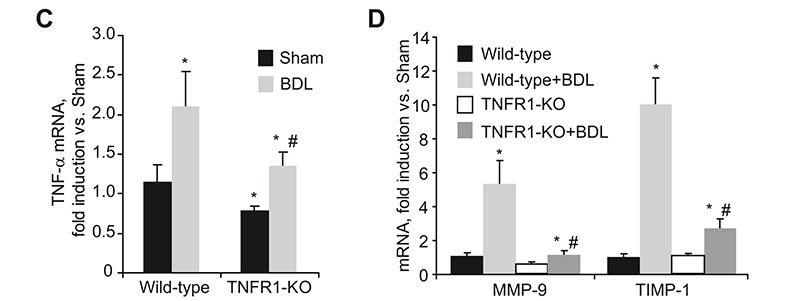
Reduced liver damage and fibrosis in TNFR1 KO mice after bile duct ligation. H&E staining of liver sections (A) and serum transaminase levels (B) in wild-type and TNFR1-KO mice after BDL. TNF-á (C), MMP-9 and TIMP-1 (D), MMP-2 and pro-Colá1(I) (E), and á-SMA (F) mRNA expression in wild-type and TNFR1-KO livers after BDL.
Liver fibrosis is the excessive accumulation of extracellular matrix proteins including collagen that occurs in most types of chronic liver diseases. Advanced liver fibrosis results in cirrhosis, liver failure, and portal hypertension and often requires liver transplantation. Activated hepatic stellate cells (HSCs) have been identified as the major collagen-producing cell-type in the injured liver. While TNF has been implicated in the progression of many chronic liver diseases leading to fibrosis; its role in fibrogenesis is controversial and the specific contribution of TNF receptors to HSCs activation remains to be established. In this study we describe, by using hepatic stellate cells (HSC) from wild-type, TNF-receptor-1 (TNFR1) knockout, TNF-receptor-2 (TNFR2) knockout, or TNFR1/R2 double knockout (TNFR-DKO) mice, that loss of both TNF receptors reduced pro-Collagen-á1(I) expression, slowed down HSC proliferation, and impaired PDGF-induced pro-mitogenic signaling in HSC. Moreover, matrix metalloproteinase-9 expression in HSCs was controlled by TNF, through TNFR1 and in vivo liver damage and fibrosis following bile duct ligation was reduced in TNFR-DKO and TNFR1 knockout mice compared to wild-type or TNFR2 knockout mice. Thus, indicating a regulatory role for TNF in extracellular matrix remodeling and liver fibrosis, suggesting that targeting TNFR1 may be of benefit to attenuate liver fibrogenesis. "For details please see Tarrats et al, Hepatology 2011 Apr 26. doi: 10.1002/hep.24388. [Epub ahead of print]".