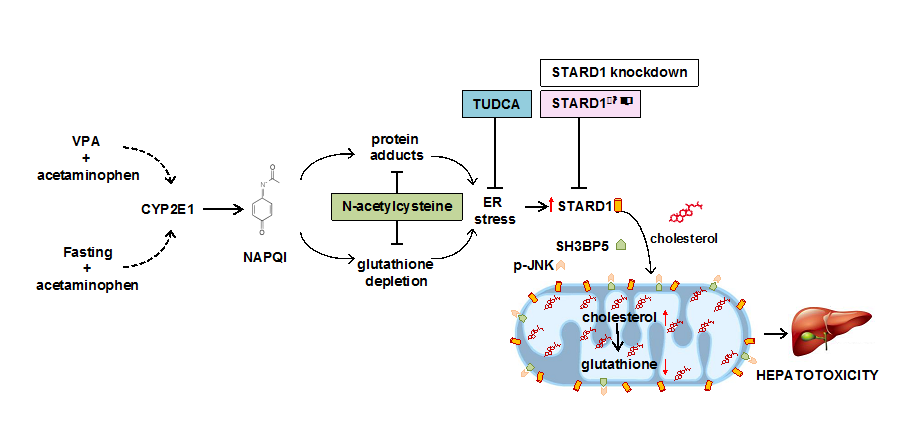
Paracetamol is one of the most consumed analgesics in the world. Although at therapeutic doses it is relatively safe, paracetamol induces hepatotoxic effects at increasing doses and is one of the main causes of fulminant hepatic failure. The mechanisms involved in the hepatotoxicity of paracetamol are not completely known, although mitochondrial dysfunction plays an important role in this process.
Now, a study carried out by the research group "Mitochondrial regulation of cell death" published in Gastroenterology with Sandra Torres as the first signatory and coordinated by Carmen Garcia Ruiz and Jose C Fernandez-Checa, has identified a new actor in the mitochondrial scene which plays a fundamental role in the hepatotoxicity induced either by paracetamol by itself or in combination with valproic acid (VPA), an antiepileptic. STARD1 is a cholesterol transporter in the mitochondria and its action determines an accumulation of cholesterol in the inner mitochondrial membrane, which leads to the disturbance of its functional properties and the limitation of mitochondrial antioxidant defense. The generation of mice with genetic deletion of STARD1, specifically in hepatocytes, has made it possible to establish the resistance of these mice to paracetamol toxicity with or without VPA. The action of STARD1 in mitochondria complements and determines the role of SAB and JNK, two known mitochondrial proteins that intervene in cellular stress, in the hepatotoxicity of paracetamol. The induction of STARD1 by paracetamol / VPA is carried out by stress in the endoplasmic reticulum that is generated from the metabolism of paracetamol in the liver. The translational relevance of these observations is found using a mouse (FRGN) with a humanized liver. These studies, therefore, demonstrate a key role of STARD1 in paracetamol hepatoxicity, emerging as a new therapeutic target.
https://www.gastrojournal.org/article/S0016-5085(19)36716-2/pdf