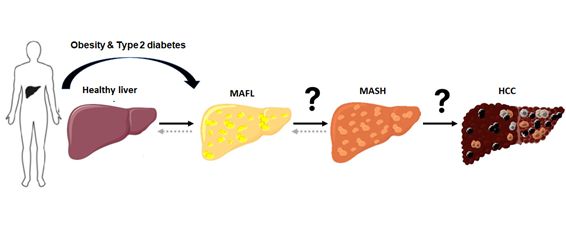
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and the end-stage of chronic liver disease that include alcoholic liver disease, viral hepatitis (HBV and HCV) and metabolic-dysfunction associated steatohepatitis (MASH), an advanced form of metabolic-dysfunction associated steatotic liver disease (MASLD). While the contribution of HBV and HCV to HCC is declining due to the availability of effective vaccines and antiviral drugs, respectively, the incidence of MASH-driven HCC is expected to continue rising throughout the world due to its association with the obesity and type-2 diabetes (T2D) epidemic. Overweight (body mass index >25) and obesity are known risk factors for cancer development, especially HCC. HCC has a poor prognosis with frequent recurrence and intrahepatic metastasis. Despite important improvements in HCC management, effective treatment options, such as local ablative therapies, resection or transplantation are mainly limited to early disease stages. Unfortunately, the therapeutic armamentarium for HCC is limited, ineffective and subject to secondary or acquired chemoresistance by poorly understood mechanisms. This is due to our incomplete understanding of the molecular events involved in HCC pathogenesis. Hence, it is urgent to understand the biochemical and molecular basis for HCC progression that will lead to the identification of new therapeutic targets for the prevention of HCC initiation and progression.
The National Cancer Institute (NCI) of the National Institutes of Health (NIH, USA) has allocated a 10.5M US dollars budget (13.8M total costs) to fund a program project grant (PO1) with investigators from the University of California San Diego (UCSD) led by Dr. Michael Karin, the Sanford Burnham Prebys Medical Discovery Institute (SBP) of San Diego led by Dr. Randal Kaufman, the University of Southern California (USC, Los Angeles) led by Dr. Neil Kaplowitz, and the IDIBAPS led by Jose C Fernandez-Checa along with Carmen Garcia Ruiz, group leaders of the IIBB-IDIBAPS, to identify new therapeutic targets for the treatment of MASH-driven HCC. Using common translational relevant experimental models, human PDXs and state-of-the art technologies (e.g. spatial transcriptomics, single nuclear RNA seq, high-resolution ultrasound imaging system, etc), the coordinated action of these research projects are expected to provide novel information about the impact of nutrient and genetic intersections in the development of MASH-HCC and novel therapeutics. The research project led by CGR and JCFC in the IIBB-IDIBAPS will be complemented by close interaction with USC to focus on the role of the STARD1-SAB axis, two resident proteins of the mitochondrial outer membrane, in metabolic stress induced mitochondrial dysfunction leading to HCC. The research of USCD, SBP and USC/IDIBAPS investigators will be supported by an administrative core with an EAB committee composed of international recognized experts, a metabolomic core and a bioinformatics, biostatistics and genomic core. Thus, it is anticipated that integration of this coordinated projects and cores will advance our understanding of MASH-HCC pathogenesis, one of the main scourges of our modern era, that will lead to the identification of new therapeutics targets for treatment.
Grant National Cancer Institute-NIH: PO1 CA282819
Title: Role of hypernutrition and metabolic stress in non-alcoholic steatohepatitis (NASH) driven hepatocellular carcinoma (HCC)
Project period: 09/09/2024-08/31/2029