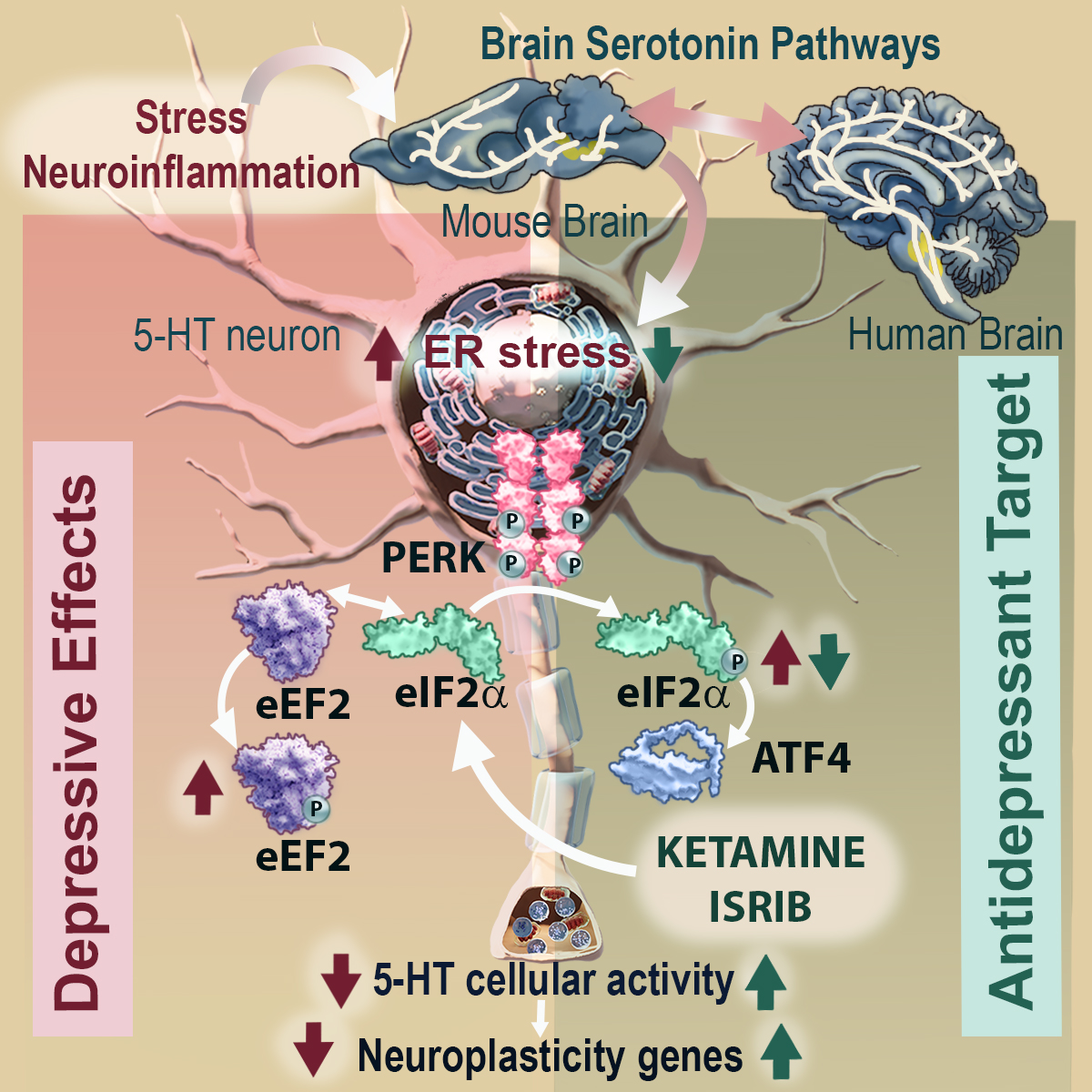
However, treatment with the fast-acting antidepressant ketamine reverses depressive symptoms by acting directly on the eIF2α signaling pathway in these neurons.
Researchers at the IIBB - CSIC have identified that endoplasmic reticulum (ER) stress in serotonin neurons is a cellular pathway involved in the pathophysiology of depression and show that the regulation of eIF2α is critical in triggering the rapid antidepressant effect of ketamine.
The journal iScience, from Cell Press, publishes the work, led by Analia Bortolozzi, scientist at the IIBB - CSIC and CIBERSAM - ISCIII and principal investigator of the Systems Neuropharmacology group at IDIBAPS.
Major depressive disorder (MDD) is a critical global mental health challenge and the leading cause of mental health-related disability worldwide. MDD is a highly prevalent mood disorder that negatively affects education, relationships and employment, and is prospectively associated with obesity, cardiovascular disease and premature death, including suicide. The disorder involves depressed mood, energy changes, sleep disturbances, poor concentration and lack of interest or arousal. Neuroimaging studies have identified structural and functional brain changes including volume reductions in cortical and subcortical structures, enlarged lateral ventricles and white matter microstructural differences suggesting compromised myelin integrity. Histopathological studies have shown changes in the density of neurons and glia, and reduced expression of synaptic proteins. Thus, all these findings converge that synaptic plasticity is key in the pathogenesis of TDD, although the pathophysiological mechanisms remain largely unknown.
In recent years, cellular stress has been suggested to contribute to the pathogenesis of complex diseases such as cancer, diabetes and metabolic disorders, but also in cognitive and neuropsychiatric disorders. Indeed, it has been hypothesised that cellular stress linked to psychosocial stressors may be a major contributor to the progression of MDD. Within eukaryotic cells (animals, humans), the main organelle showing an adaptive response to cellular stress is the endoplasmic reticulum (ER). Under normal conditions, the ER regulates multiple biological processes such as the homeostatic maintenance of proteins for proper cellular function, such as the regulation of synaptic proteins in cognitive and motor function. However, under stress signals, neuroinflammation, changes in intracellular calcium, among others, ER dysregulation is induced, known as ER stress.
In this study, we focused on exploring whether the intracellular PERK-eIF2α signaling pathway in serotonin neurons, in the context of ER stress, contributes to the depressive phenotype. To do so, we induced mouse models that develop ER stress in serotonin neurons located in the midbrain region. The results showed that phosphorylation of the eIF2α factor is a key mediator of 5-HT-dependent neuroplasticity processes and strongly support it as a site of antidepressant action of ketamine, says Dr Bortolozzi.
Dr Miquel-Rio, first author of the paper, comments that when ER stress is induced in these neurons, the mice develop a depressive-anxious phenotype when compared to normal mice. In parallel, the mice showed reduced serotonergic neurotransmission and expression of different neuroplasticity genes in brain areas such as the prefrontal cortex, hippocampus and amygdala, which are directly related to emotion control. Treatment with the antidepressant ketamine mitigates local ER stress and reverses the depressive-anxious phenotype.
The study has also shown that the eIF2α signaling pathway plays a key role in the antidepressant mechanism of action of ketamine. Under conditions of selective blockade of the eIF2α-GADD34:PP1 pathway, the antidepressant effect of ketamine disappears. Bortolozzi comments that the eIF2α factor may be a new target for antidepressant action. In fact, the agent ISRIB, known as the miracle molecule to treat virtually any neuronal ailment, from Alzheimer's to brain damage, may even be able to rejuvenate the brain and stimulate memory, blocked the effects of eIF2α phosphorylation, which led to antidepressant effects in the mouse models used in the study.
Scientists from the Institute of Biomedicine and Biotechnology of Cantabria (IIBBTEC), the Centre for Biomedical Research in Mental Health Network (CIBERSAM) and miCure Therapeutics Biotechnology (Tel-Aviv, Israel) have also participated in the study.
Reference article
Miquel-Rio L, Sarriés-Serrano U, Sancho-Alonso M, Florensa-Zanuy E, Paz V, Ruiz-Bronchal E, Manashirov S, Campa L, Pilar-Cuéllar F, Bortolozzi A. ER stress in mouse serotonin neurons triggers a depressive phenotype alleviated by ketamine targeting eIF2α signaling. iScience. 2024 Apr 18;27(5):109787. doi: 10.1016/j.isci.2024.109787. PMID: 38711453; PMCID: PMC11070602.