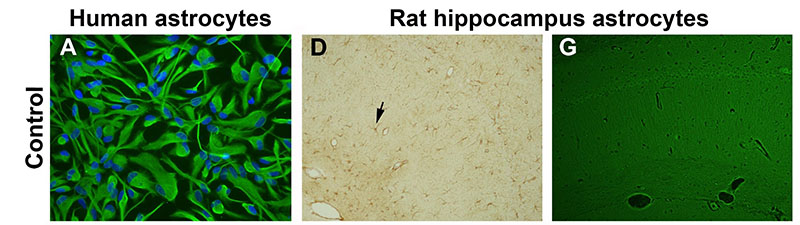
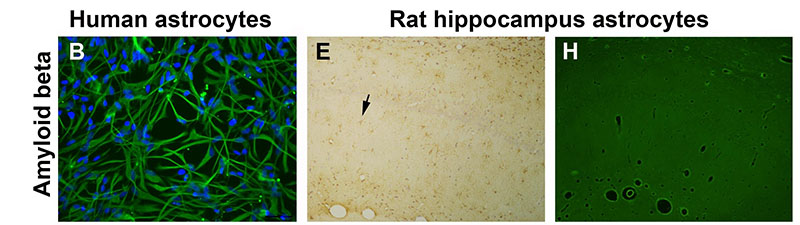
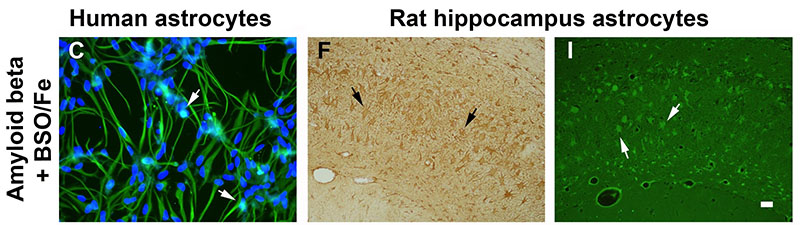
Pro-oxidant conditions trigger the toxic and inflammatory effects of amyloid beta in astrocytes. A-C) Human astrocyte cultures control, treated for 3 days with amyloid beta 42, and treated with amyloid beta and the prooxidant agents buthionine sulfoximine (BSO) and ferrous sulfate (Fe), respectively. BSO was added 24 h before the other agents. Astrocytes were stained with anti-GFAP antibody and nuclei were counterstained with bisbenzimide. Apoptotic nuclei show a bright fluorescence (arrows). Treatment with amyloid beta alone caused a slight change to an activated morphology. However, the mixed treatment with amyloid beta and pro-oxidant agents caused astrocyte activation and apoptosis. D-I) Hippocampus Ammon’s horn of rats submitted to 4 weeks of chronic icv infusion with vehicle, amyloid beta 40 and 42, and amyloid beta 42 and BSO and Fe. Immunohistochemical stain with anti-GFPA antibody and light counterstain with hematoxylin (D-F) and Fluoro-Jade B fluorescence staining (G-I), show morphological changes and reactivity of astrocytes (arrows). The higher astrocyte reactivity was caused by amyloid beta and prooxidant agents, where astrocytes also stained positive for Fluoro-Jade B. For details of treatment groups and dosing see full text. Scale bar = 20 ?m in A-C and 40 ?m in D-I.
Alzheimer’s disease (AD) is the leading cause of dementia in people over 65. No cure has yet been found and current pharmacological therapy only temporarily ameliorates the symptoms. Age is the main risk factor and a better understanding of age-related changes in the brain cells will help fight AD. The authors previously reported that aged astrocytes suffer oxidative stress and inflammation and their neuroprotective function is reduced. In this work, in vitro human astrocytes and in vivo rats were treated with prooxidant agents (buthionine sulfoximine, a glutathione synthesis inhibitor, and reactive iron formulated as ferrous sulfate) to mimic the cell environment in the aged brain. Either prooxidant agents or amyloid beta peptide did not cause deleterious effects in the astrocytes, but the combined treatment let to oxidative stress and apoptosis in vitro and inflammation and degenerative traits in vivo. Therefore, a reduced oxidative stress defense capacity in frail aged astrocytes may contribute to neuron death by failure of astrocyte support. To preserve astrocyte function in old age is a new goal against AD. Full article published by García-Matas et al., Journal of Alzheimer’s Disease 2010, Apr;20(1):229-45.