Cholestatic liver diseases (CLD) are often accompanied by hepatocellular injury, fibrosis, and cirrhosis due to the intracellular accumulation of solutes that cannot be excreted into bile, including bile acids (BAs), which when accumulated in the liver induce liver failure by ill understood mechansisms. Therapeutic treatment for CLD are available but limited as the biochemical response to first-line therapy with ursodeoxycholic acid is associated with major complications in about 40% of patients with primary biliary colangitis (PBC), a rare-immune mediated form of CLD.
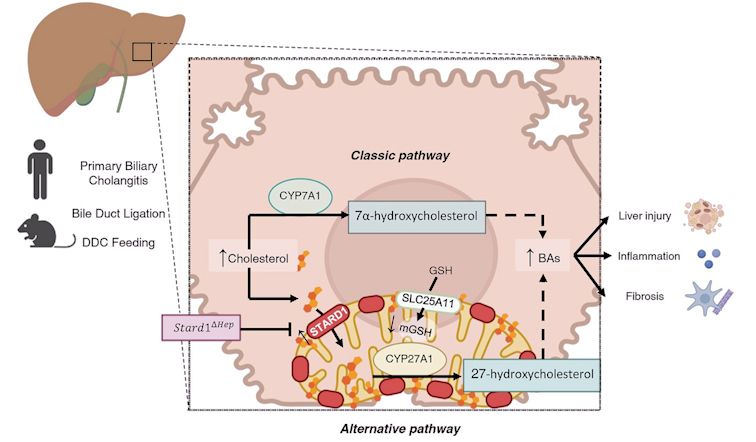
Using samples from patients with PBC and experimental models of complete (bile duct ligation model, BDL) and chemical-induced obstructive cholestasis (DCC feeding), work from the group Mitochondrial Regulation of Cell Death, led by Jose C. Fernandez-Checa and Carmen García Ruiz and investigators from University of Salamanca, with Laura Conde de la Rosa and Laura Fàbrega as first authors demonstrates a crucial role for STARD1 in hepatocellular injury, inflammation and fibrosis, which thus entails the progression of CLD. Patients with PBC exhibit increased expression of hepatic STARD1 levels, and genetic deletion of Stard1 in hepatocytes in mice protects from bile duct ligation (BDL)-induced cholestatic liver injury and disease progression.
The classic pathway of BAs synthesis is considered the main source of BAs generation in the adult liver, as CYP7A1 expression increases after weaning. Unlike the classic pathway in which expression of CYP7A1 is the regulatory step, the mitochondrial alternative pathway of BAs synthesis is controlled by the availability of cholesterol in the mitochondrial inner membrane, which is governed by STARD1.
The induction of STARD1 in CLD causes two important events, the stimulation of BAs synthesis via the alternative pathway, and the depletion of mitochondrial GSH due to cholesterol accumulation and subsequent decrease in membrane fluidity, which sensitizes primary hepatocytes to BAs-induced cytotoxicity and inflammatory response. The in vivo findings in BDL were reproduced in vitro in primary cultured mouse hepatocytes with pharmacological depletion of mitochondrial GSH, which unmasked their sensitivity to BA-induced cytotoxicity and inflammation as well as DNA damage revealed by the comet tail lenght. Thus, our findings identify a previously unrecognized role for STARD1 in cholestasis and suggest that targeting STARD1 may be a novel approach for CLD.
Reference of the article:
Conde de la Rosa L, Fábrega L, Torres S, Nuñez S, Ribas V, Segalés P, Espinosa-Escudero R, Solsona E, Monte MJ, Diaz-Gonzalez A, Marin JJG, García-Ruiz C, Fernandez-Checa JC. Stard1 promotes cholestatic liver injury and disease progression by sensitizing to bile acid hepatotoxicity. HEPATOLOGY 2024 Dec 9. doi: 10.1097/HEP.0000000000001184.