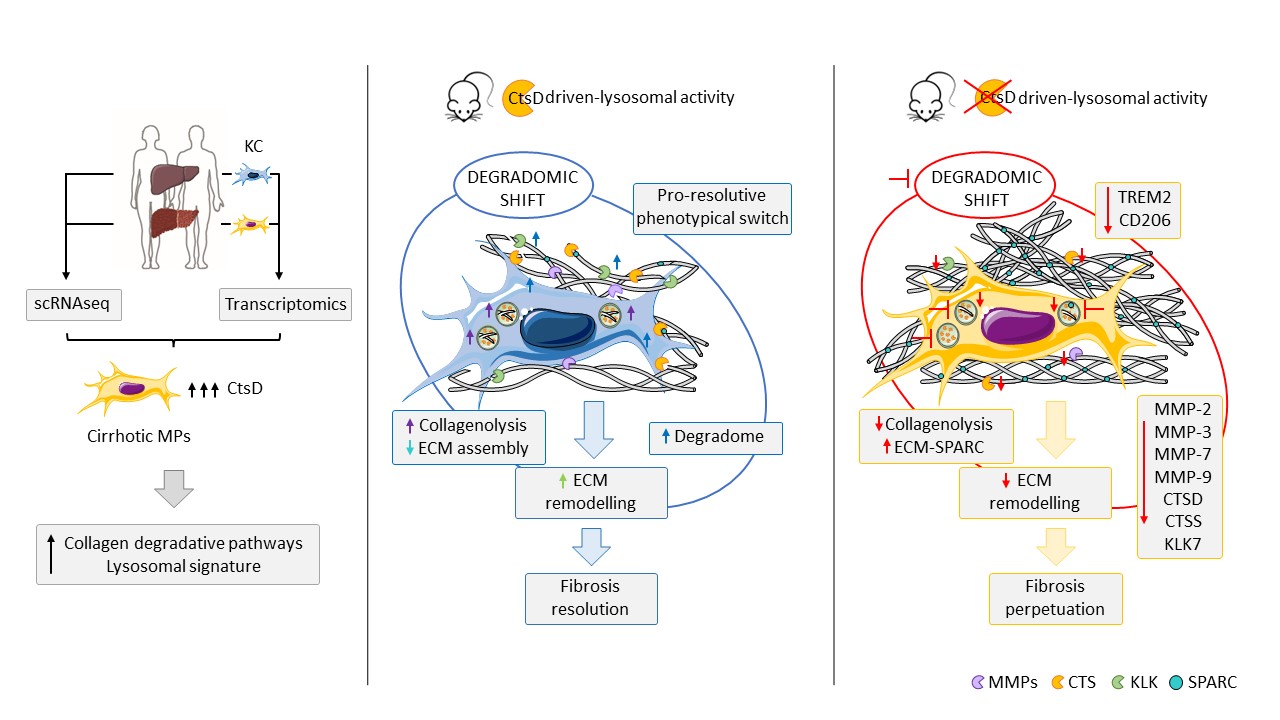
The Tissue Remodelling, Fibrosis and Cancer group led by Dr Anna Moles publishes a new original research article in the journal Molecular Metabolism entitled "Cathepsin D is essential for the degradomic shift of macrophages required to resolve liver fibrosis". Fibrosis contributes to 45% of deaths in industrialized nations and is characterized by an abnormal accumulation of extracellular matrix. Currently there are no specific antifibrotic treatments for liver fibrosis. Previous failed attempts at drug development have focused on preventing extracellular matrix deposition. Since liver fibrosis is reversible, regulating fibrosis resolution could offer new therapeutic options. However, little is known about the mechanisms that control extracellular matrix remodeling during liver fibrosis resolution.
Our work sheds new light on how macrophages contribute to the resolution of liver fibrosis. In this work we describe how the lysosomal protease CtsD contributes to the degradomic switch of macrophages, which comprises secretomic, phenotypic and functional changes, necessary for macrophages to proteolytically process the extracellular matrix during liver fibrosis resolution. Our work identifies for the first time CtsD-driven lysosomal activity as a central hub for restorative macrophage function during liver fibrosis and proposes to explore the proteolytic profile of macrophages for the development of new antifibrotic drugs.
This work has been funded by the Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033/FEDER, EU (projects: PID2021-123652OB-I00, RTI2018-097475-A-100 and contracts: RYC-2016-19731 and PRE2022-101676), Pfizer (project: #77131383), the Ministry of Universities (contracts: FPU19/05357 and FPU20/01367) and the CSIC (contract: JAEINT_23_0124).